Abstract
Background: Patients (pts) with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) or mantle cell lymphoma (MCL) have an increased risk of major hemorrhage (MH) compared with the age- and sex-matched general population (Gifkins ASH 2015; abstract 3268). Ibrutinib (ibr), a first-in-class, once-daily inhibitor of Bruton's tyrosine kinase, is approved globally for the treatment of various B-cell malignancies. Early clinical studies with ibr showed increased rates of low-grade hemorrhage (most commonly contusion, epistaxis, and petechiae) and infrequently, serious hemorrhage, which led to the inclusion of risk of hemorrhage in the Warnings and Precautions section of the ibr US and EU prescribing information. An integrated analysis was conducted to characterize the risks of MH with ibr, and to evaluate the potential association of MH with concomitant use of antiplatelet (AP) and/or anticoagulant (AC) agents.
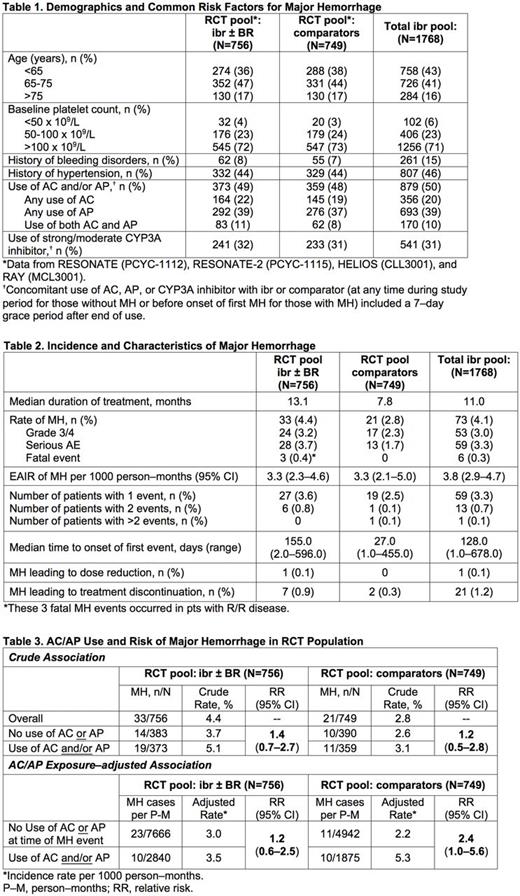
Methods: Data were analyzed from 4 randomized controlled trials (RCT) of pts with treatment-naïve (TN) or relapsed/refractory (R/R) CLL/SLL and MCL: RESONATE (ibr vs ofatumumab in R/R CLL/SLL), RESONATE-2 (ibr vs chlorambucil in older TN CLL/SLL), HELIOS (bendamustine and rituximab [BR] ± ibr in R/R CLL/SLL), and RAY (ibr vs temsirolimus in R/R MCL). These RCT pools comprised 756 ibr-treated pts and 749 comparator-treated pts. An additional analysis included 1768 ibr-treated pts (total ibr pool, which includes the 4 RCTs above) with B-cell malignancies (CLL/SLL, MCL, diffuse large B-cell lymphoma, marginal zone lymphoma, follicular lymphoma, and Waldenström's macroglobulinemia) from completed clinical studies of single-agent or combination regimens with ibr. MH was defined as preferred terms in the sub-Standardized MedDRA Query "Hemorrhage terms (excluding laboratory terms)": grade ≥3 adverse events (AEs), serious AEs (SAEs), and any grade CNS hemorrhage.
Results: Baseline characteristics were similar between RCT pools (Table 1), but median treatment duration was longer with ibr vs comparators (13.1 vs 7.8 mo). Characteristics in the RCT ibr pool were also similar to those of the total ibr pool, except nearly twice the proportion of pts in the total ibr pool had a history of bleeding disorders (Table 1). In the RCT pool, the proportion of MH was higher with ibr (4.4%) vs comparator (2.8%); however, given the longer exposure to ibr, the exposure-adjusted incidence rate (EAIR) was similar between RCT ibr and comparator pools (3.3 per 1000 person-months; Table 2). In the total ibr pool, the proportion of MH was 4.1% and EAIR was 3.8 per 1000 person-months. In the RCT pool, most pts with MH had 1 MH event, with longer median time to onset with ibr vs comparators. Grade 3/4 MH and SAE events of MH occurred in 3.2% and 3.7% in the RCT ibr pool, respectively, vs 2.3% and 1.7% in RCT comparators. Fatal MH occurred in 3 pts in the RCT ibr pool (1 case each of subdural hematoma, post-procedural hemorrhage, and aortic aneurysm) vs none in comparators (Table 2). By univariate analysis, use of AC and/or AP (versus no AC/AP) was similarly associated with increased risk of MH in both ibr and comparators in the RCT pool (Table 3). AC/AP exposure-adjusted relative risk was 1.2 (95% CI: 0.6-2.5) in RCT ibr pool, 2.4 (95% CI: 1.0-5.6) in RCT comparators, and 1.9 (95% CI: 1.2-3.0) in total ibr pool. Among 311 ibr-treated pts exposed to any AC (mean of 94 days; median 16 days [range, 1-667] given use of LMWH/heparin in 89%) in total ibr pool, MH occurred in 20 pts (6.4%). Among 677 ibr-treated pts exposed to any AP (mean of 230 days; median 174 days [range, 1-982]) in total ibr pool, MH occurred in 30 pts (4.4%). In the total ibr pool, MH led to dose reduction in 1 pt (<1%) and discontinuation in 21 pts (1%); among 20 CNS MH, 13 discontinued, whereas among 53 non-CNS MH, 8 discontinued.
Conclusions: Results of this integrated analysis show that the proportion of pts with MH was higher with ibr than with comparators, but after adjusting for treatment exposure, the rate of MH was similar between ibr and comparators. AC and/or AP use occurred in approximately half of all pts on ibr studies, although the duration of AC use in our clinical trials may not be reflective of AC use in the real-world practice setting. Use of AC and/or AP was associated with MH in both ibr and comparators with a similar degree of association, suggesting that ibr therapy does not alter the effect of AC or AP on MH risk. MH leading to ibr discontinuation occurred in 1% of ibr-treated pts.
Brown: Gilead Sciences: Consultancy, Research Funding; AbbVie: Consultancy, Honoraria; Janssen Oncology: Honoraria; Pharmacyclics: Consultancy; Janssen: Consultancy; Roche/Genentech: Consultancy; Celgene: Consultancy; Sun Pharma: Consultancy; Infinity Pharmaceuticals: Consultancy; AstraZeneca: Consultancy; Redx: Consultancy; Astellas Pharma: Consultancy. Moslehi: Takeda: Consultancy; Pfizer: Consultancy; Bristol-Myers Squibb: Consultancy; Ariad: Consultancy; Vertex: Consultancy; Novartis: Consultancy; Pharmacyclics: Consultancy; Daiichi Sankyo: Consultancy; Incyte: Consultancy; Regeneron: Consultancy; Acceleron: Consultancy; Heat Biologics: Consultancy; Verastem: Consultancy; Rgenix: Consultancy; StemCentRx: Consultancy. Ewer: Pharmacyclics: Honoraria; Bohringer-Ingelheim: Consultancy; Astra Zeneca: Consultancy; Roche: Consultancy; People's Publishing: Patents & Royalties: Cancer and the Heart (textbook); Pharmacyclics: Other: Travel, Accommodations, Expenses. O'Brien: CLL Global Research Foundation: Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy; Janssen: Consultancy; Pharmacyclics: Consultancy, Other: Research Support: Honorarium, Research Funding; Astellas: Consultancy; ProNAI: Other: Research Support: Honorarium, Research Funding; Gilead Sciences, Inc.: Consultancy, Other: Research Support: Honorarium, Research Funding; Amgen: Consultancy; Vaniam Group LLC: Consultancy; Celgene: Consultancy; GSK: Consultancy; Acerta: Other: Research Support: Honorarium, Research Funding; Sunesis: Consultancy; Aptose Biosciences, Inc.: Consultancy; Pfizer: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: Research Support: Honorarium, Research Funding; AbbVie: Consultancy; Regeneron: Other: Research Support: Honorarium, Research Funding. Ghia: Roche: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Novartis: Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; Adaptive: Consultancy. Cymbalista: AbbVie: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Mundipharma: Honoraria; Gilead: Consultancy, Honoraria; Roche: Other: Travel, Accommodations, Expenses. Shanafelt: GlaxoSmithKline: Research Funding; Pharmacyclics: Research Funding; Celgene: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Mayo Clinic: Patents & Royalties: Co-inventor of the Physician Well-being Index, Medical Student Well-Being Index, and Well-being Index. Mayo Clinic holds the copyright for this instrument and licensed it for use outside of Mayo Clinic. I receive a portion of any royalties paid to Mayo.; Cephalon: Research Funding; Hospira: Research Funding. Fraser: Celgene: Research Funding; Lundbeck: Speakers Bureau; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy. Rule: Napp: Consultancy; TG Therapeutics: Consultancy, Honoraria; Sunesis: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Kite: Consultancy; Astra-Zeneca: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding. Coutre: Abbvie: Consultancy, Research Funding; Gilead: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy. Dilhuydy: Roche: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses. Cramer: Gilead: Other: travel support, Research Funding; AbbVie: Consultancy; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding; Novartis: Consultancy, Research Funding; F. Hoffmann-LaRoche: Honoraria, Other: travel support, Research Funding; AstraZeneca: Consultancy; GSK: Research Funding. Jaeger: Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Novartis Pharmaceuticals Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses. Dreyling: Bayer: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Roche: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Mundipharma: Research Funding. Byrd: Genentech: Research Funding; Acerta: Research Funding; Pharmacyclics: Research Funding. Treon: Pharmacyclics, Inc.: Consultancy, Research Funding; Janssen Pharm.: Consultancy. Liu: AbbVie: Equity Ownership, Other: Travel, Accommodations, Expenses; Theorem Clinical Research: Employment; Pharmacyclics: Employment. Chang: Pharmacyclics: Employment; AbbVie: Equity Ownership; JNJ: Equity Ownership; Portola: Equity Ownership. Bista: Pharmacyclics LLC: Employment; AbbVie: Equity Ownership. Vempati: Pharmacyclics LLC: Employment, Other: Travel, Accommodations, Expenses; AbbVie: Equity Ownership. Boornazian: Pharmacyclics: Employment; GlaxoSmithKline: Employment, Equity Ownership; AstraZeneca: Employment, Equity Ownership; AbbVie: Equity Ownership; OncoGenex: Equity Ownership. Valentino: Gilead: Equity Ownership; Pharmacyclics: Employment, Membership on an entity's Board of Directors or advisory committees; AbbVie: Equity Ownership. Reddy: Pharmacyclics: Employment, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; Janssen JNJ: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses; AbbVie: Equity Ownership. Mahler: Johnson&Johnson: Employment, Equity Ownership. Yang: Pharmacyclics: Employment; AbbVie: Equity Ownership. Burger: Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Novartis: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal